Altered interactions between circulating and tissue-resident CD8 T cells with the colonic mucosa define colitis associated with immune checkpoint inhibitors
Molly Fisher Thomas*, Kamil Slowikowski*, Kasidet Manakongtreecheep, Pritha Sen, Jessica Tantivit, Mazen Nasrallah, Neal P. Smith, Swetha Ramesh, Leyre Zubiri, Alice Tirard, Benjamin Y. Arnold, Linda T. Nieman, Jonathan H. Chen, Thomas Eisenhaure, Karin Pelka, Katherine H. Xu, Vjola Jorgji, Christopher J. Pinto, Tatyana Sharova, Rachel Glasser, Elaina PuiYee Chan, Ryan J. Sullivan, Hamed Khalili, Dejan Juric, Genevieve M. Boland, Michael Dougan, Nir Hacohen, Kerry L. Reynolds, Bo Li, Alexandra-Chloé Villani
bioRxiv, 2021. DOI: 10.1101/2021.09.17.460868
Abstract
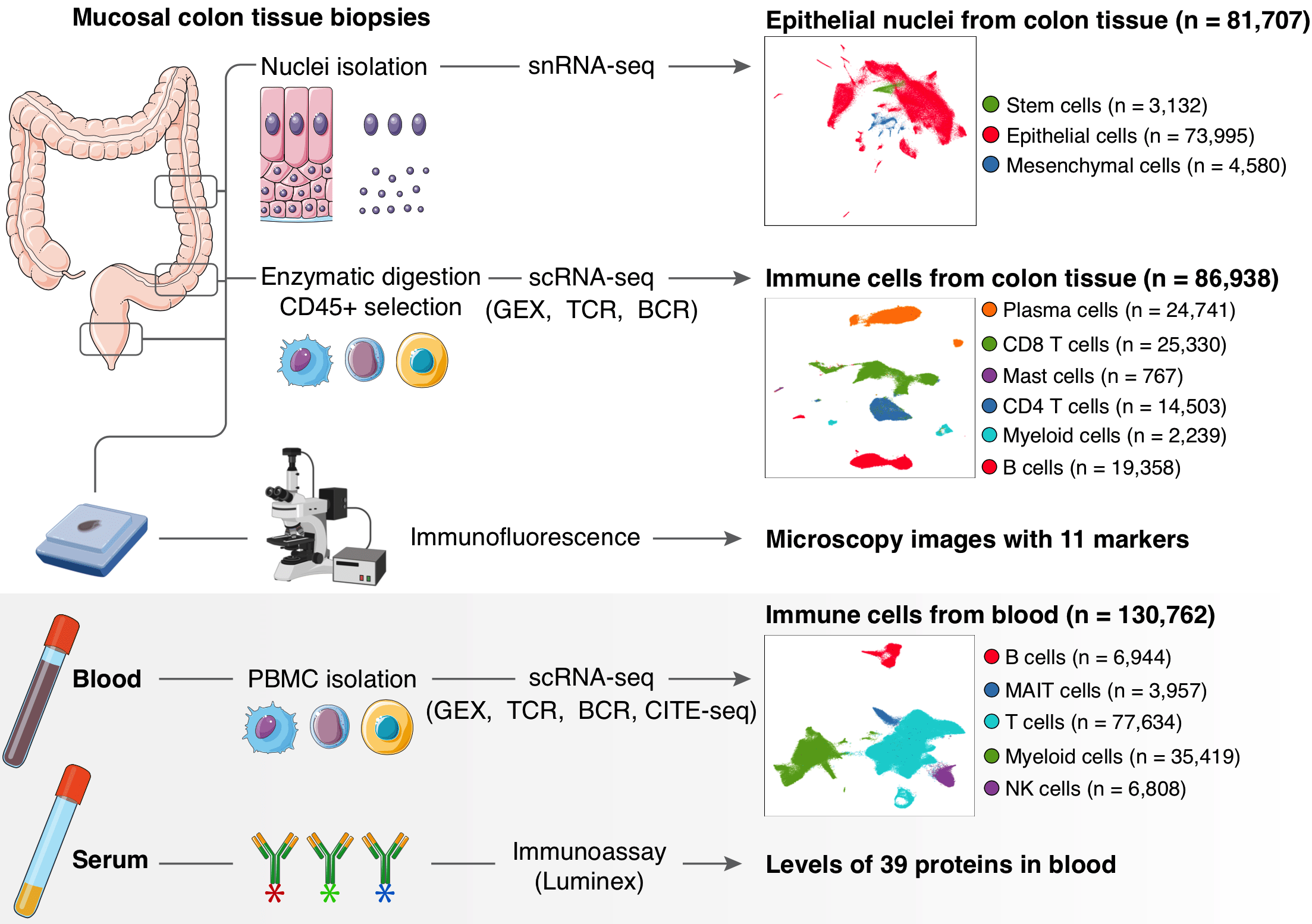
Therapeutic blockade of co-inhibitory immune receptors PD-1 and CTLA-4 has revolutionized oncology, but treatments are limited by immune-related adverse events (IRAEs). IRAE Colitis (irColitis) is the most common, severe IRAE affecting up to 25% of patients on dual PD-1 and CTLA-4 inhibition. Here, we present a systems biology approach to define the cell populations and transcriptional programs driving irColitis. We collected paired colon mucosal biopsy and blood specimens from 13 patients with irColitis, 8 healthy individuals, and 8 controls on immune checkpoint inhibitors (ICIs), and analyzed them with single-cell/nuclei RNA sequencing with paired TCR and BCR sequencing, multispectral fluorescence microscopy, and secreted factor analysis (Luminex). We profiled 299,407 cells from tissue and blood and identified 105 cell subsets that revealed significant tissue remodeling in active disease. Colon mucosal immune populations were dominated by tissue-resident memory (TRM) ITGAE-expressing CD8 T cells representing a phenotypic spectrum defined by gene programs associated with T cell activation, cytotoxicity, cycling, and exhaustion. CD8 TRM and effector CD4 T cells upregulated type 17 immune programs (IL17A, IL26) and Tfh-like programs (CXCL13, PDCD1). We also identified for the first time an increased abundance of two KLRG1 and ITGB2-expressing CD8 T cell populations with circulatory cell markers, including a GZMK TRM-like population and a CX3CR1 population that is predicted to be intravascular. These two populations were more abundant in irColitis patients treated with dual PD-1/CTLA-4 inhibition than those receiving anti-PD-1 monotherapy. They also had significant TCR sharing with PBMCs, suggesting a circulatory origin. In irColitis we observed significant epithelial turnover marked by fewer LGR5-expressing stem cells, more transit amplifying cells, and upregulation of apoptotic and DNA-sensing programs such as the cGAS-STING pathway. Mature epithelial cells with top crypt genes upregulated interferon-stimulated pathways, CD274 (PD-L1), anti-microbial genes, and MHC-class II genes, and downregulated aquaporin and solute-carrier gene families, likely contributing to epithelial cell damage and absorptive dysfunction. Mesenchymal remodeling was defined by increased endothelial cells, both in irColitis patients and specifically in patients on dual PD-1/CTLA-4 blockade. Cell-cell communication analysis identified putative receptor-ligand pairs that recruit CD8 T cells from blood to inflamed endothelium and positive feedback loops such as the CXCR3 chemokine system that retain cells in tissue. This study highlights the cellular and molecular drivers underlying irColitis and provides new insights into the role of CTLA-4 and PD-1 signaling in maintaining CD8 TRM homeostasis, regulating CD8 T recruitment from blood, and promoting epithelial-immune crosstalk critical to gastrointestinal immune tolerance and intestinal barrier function.