Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis
Fumitaka Mizoguchi*, Kamil Slowikowski*, Kevin Wei, Jennifer L. Marshall, Deepak A. Rao, Sook Kyung Chang, Hung N. Nguyen, Erika H. Noss, Jason D. Turner, Brandon E. Earp, Philip E. Blazar, John Wright, Barry P. Simmons, Laura T. Donlin, George D. Kalliolias, Susan M. Goodman, Vivian P. Bykerk, Lionel B. Ivashkiv, James A. Lederer, Nir Hacohen, Peter A. Nigrovic, Andrew Filer, Christopher D. Buckley, Soumya Raychaudhuri & Michael B. Brenner
Nature Communications, 2018. DOI: 10.1038/s41467-018-02892-y
Abstract
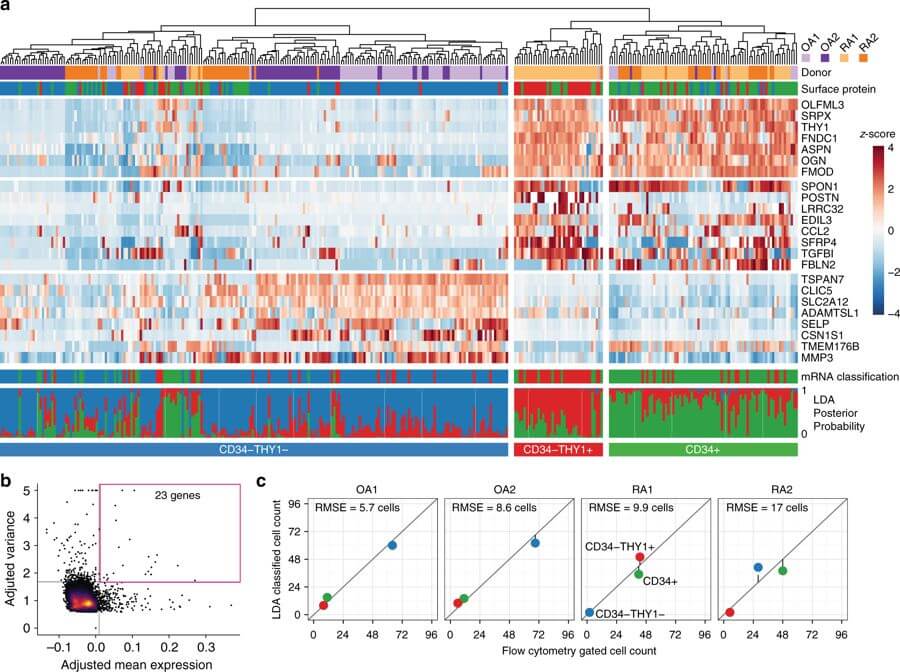
Fibroblasts regulate tissue homeostasis, coordinate inflammatory responses, and mediate tissue damage. In rheumatoid arthritis (RA), synovial fibroblasts maintain chronic inflammation which leads to joint destruction. Little is known about fibroblast heterogeneity or if aberrations in fibroblast subsets relate to pathology. Here, we show functional and transcriptional differences between fibroblast subsets from human synovial tissues using bulk transcriptomics of targeted subpopulations and single-cell transcriptomics. We identify seven fibroblast subsets with distinct surface protein phenotypes, and collapse them into three subsets by integrating transcriptomic data. One fibroblast subset, characterized by the expression of proteins podoplanin, THY1 membrane glycoprotein and cadherin-11, but lacking CD34, is threefold expanded in patients with RA relative to patients with osteoarthritis. These fibroblasts localize to the perivascular zone in inflamed synovium, secrete proinflammatory cytokines, are proliferative, and have an in vitro phenotype characteristic of invasive cells. Our strategy may be used as a template to identify pathogenic stromal cellular subsets in other complex diseases.